Nad is Recycled Back to Nadh to Ensure Glycolytic Continuity
Humans have taken advantage of the metabolism in a tiny fungus called yeast to create beer and wine from grains and fruits. What are the biological mechanisms behind this alcohol production?
Once upon a time, many, many years ago, a man found a closed fruit jar containing a honeybee. When he drank the contents, he tasted a new, strange flavor. Suddenly his head was spinning, he laughed for no reason, and he felt powerful. He drank all the liquid in the jar. The next day he experienced an awful feeling. He had a headache, pain, an unpleasant taste in his mouth, and dizziness — he had just discovered the hangover. You might think this is just a tale, but is it? Several archaeological excavations have discovered jars containing the remains of wine that are 7,000 years old (McGovern, 2009), and it is very likely that humankind's first encounter with alcoholic beverages was by chance. How did this chance discovery lead to the development of the beer and wine industry (Figure 1), and how did scientists eventually learn about the biological mechanisms of alcohol production?
The History of Beer and Wine Production
Over the course of human history, and using a system of trial, error, and careful observation, different cultures began producing fermented beverages. Mead, or honey wine, was produced in Asia during the Vedic period (around 1700–1100 BC), and the Greeks, Celts, Saxons, and Vikings also produced this beverage. In Egypt, Babylon, Rome, and China, people produced wine from grapes and beer from malted barley. In South America, people produced chicha from grains or fruits, mainly maize; while in North America, people made octli (now known as "pulque") from agave, a type of cactus (Godoy et al. 2003).
At the time, people knew that leaving fruits and grains in covered containers for a long time produced wine and beer, but no one fully understood why the recipe worked. The process was named fermentation, from the Latin word fervere, which means "to boil." The name came from the observation that mixtures of crushed grapes kept in large vessels produced bubbles, as though they were boiling. Producing fermented beverages was tricky. If the mixture did not stand long enough, the product contained no alcohol; but if left for too long, the mixture rotted and was undrinkable. Through empirical observation, people learned that temperature and air exposure are key to the fermentation process.
Wine producers traditionally used their feet to soften and grind the grapes before leaving the mixture to stand in buckets. In so doing, they transferred microorganisms from their feet into the mixture. At the time, no one knew that the alcohol produced during fermentation was produced because of one of these microorganisms — a tiny, one-celled eukaryotic fungus that is invisible to the naked eye: yeast. It took several hundred years before quality lenses and microscopes revolutionized science and allowed researchers to observe these microorganisms.
Yeast and Fermentation

Figure 1: Fermented beverages such as wine have been produced by different human cultures for centuries.
Christian Draghici/Shutterstock. All rights reserved.
In the seventeenth century, a Dutch tradesman named Antoni van Leeuwenhoek developed high-quality lenses and was able to observe yeast for the first time. In his spare time Leeuwenhoek used his lenses to observe and record detailed drawings of everything he could, including very tiny objects, like protozoa, bacteria, and yeast. Leeuwenhoek discovered that yeast consist of globules floating in a fluid, but he thought they were merely the starchy particles of the grain from which the wort (liquid obtained from the brewing of whiskey and beer) was made (Huxley 1894). Later, in 1755, yeast were defined in the Dictionary of the English Language by Samuel Johnson as "the ferment put into drink to make it work; and into bread to lighten and swell it." At the time, nobody believed that yeast were alive; they were seen as just organic chemical agents required for fermentation.
In the eighteenth and nineteenth centuries, chemists worked hard to decipher the nature of alcoholic fermentation through analytical chemistry and chemical nomenclature. In 1789, the French chemist Antoine Lavoisier was working on basic theoretical questions about the transformations of substances. In his quest, he decided to use sugars for his experiments, and he gained new knowledge about their structures and chemical reactions. Using quantitative studies, he learned that sugars are composed of a mixture of hydrogen, charcoal (carbon), and oxygen.
Lavoisier was also interested in analyzing the mechanism by which sugarcane is transformed into alcohol and carbon dioxide during fermentation. He estimated the proportions of sugars and water at the beginning of the chemical reaction and compared them with the alcohol and carbon dioxide proportions obtained at the end. For the alcoholic reaction to proceed, he also added yeast paste (or "ferment," as it was called). He concluded that sugars were broken down through two chemical pathways: Two-thirds of the sugars were reduced to form alcohol, and the other third were oxidized to form carbon dioxide (the source of the bubbles observed during fermentation). Lavoisier predicted (according to his famous conservation-of-mass principle) that if it was possible to combine alcohol and carbon dioxide in the right proportions, the resulting product would be sugar. The experiment provided a clear insight into the basic chemical reactions needed to produce alcohol. However, there was one problem: Where did the yeast fit into the reaction? The chemists hypothesized that the yeast initiated alcoholic fermentation but did not take part in the reaction. They assumed that the yeast remained unchanged throughout the chemical reactions.
Yeast Are Microorganisms
In 1815 the French chemist Joseph-Louis Gay-Lussac made some interesting observations about yeast. Gay-Lussac was experimenting with a method developed by Nicolas Appert, a confectioner and cooker, for preventing perishable food from rotting. Gay-Lussac was interested in using the method to maintain grape juice wort in an unfermented state for an indefinite time. The method consisted of boiling the wort in a vessel, and then tightly closing the vessel containing the boiling fluid to avoid exposure to air. With this method, the grape juice remained unfermented for long periods as long as the vessel was kept closed. However, if yeast (ferment) was introduced into the wort after the liquid cooled, the wort would begin to ferment. There was now no doubt that yeast were indispensable for alcoholic fermentation. But what role did they play in the process?
When more powerful microscopes were developed, the nature of yeast came to be better understood. In 1835, Charles Cagniard de la Tour, a French inventor, observed that during alcoholic fermentation yeast multiply by gemmation (budding). His observation confirmed that yeast are one-celled organisms and suggested that they were closely related to the fermentation process. Around the same time, Theodor Schwann, Friedrich Kützing, and Christian Erxleben independently concluded that "the globular, or oval, corpuscles which float so thickly in the yeast [ferment] as to make it muddy" were living organisms (Barnett 1998). The recognition that yeast are living entities and not merely organic residues changed the prevailing idea that fermentation was only a chemical process. This discovery paved the way to understand the role of yeast in fermentation.
Pasteur Demonstrates the Role of Yeast in Fermentation

Figure 2: Louis Pasteur
Our modern understanding of the fermentation process comes from the work of the French chemist Louis Pasteur.
© 2002 Nature Publishing Group Mazzarello, P. Life out of nowhere? Nature 417, 792-793 (2002). All rights reserved. ![]()
Our modern understanding of the fermentation process comes from the work of the French chemist Louis Pasteur (Figure 2). Pasteur was the first to demonstrate experimentally that fermented beverages result from the action of living yeast transforming glucose into ethanol. Moreover, Pasteur demonstrated that only microorganisms are capable of converting sugars into alcohol from grape juice, and that the process occurs in the absence of oxygen. He concluded that fermentation is a vital process, and he defined it as respiration without air (Barnett 2000; Pasteur 1876).
Pasteur performed careful experiments and demonstrated that the end products of alcoholic fermentation are more numerous and complex than those initially reported by Lavoisier. Along with alcohol and carbon dioxide, there were also significant amounts of glycerin, succinic acid, and amylic alcohol (some of these molecules were optical isomers — a characteristic of many important molecules required for life). These observations suggested that fermentation was an organic process. To confirm his hypothesis, Pasteur reproduced fermentation under experimental conditions, and his results showed that fermentation and yeast multiplication occur in parallel. He realized that fermentation is a consequence of the yeast multiplication, and the yeast have to be alive for alcohol to be produced. Pasteur published his seminal results in a preliminary paper in 1857 and in a final version in 1860, which was titled "Mémoire sur la fermentation alcoolique" (Pasteur 1857).
In 1856, a man named Bigo sought Pasteur's help because he was having problems at his distillery, which produced alcohol from sugar beetroot fermentation. The contents of his fermentation containers were embittered, and instead of alcohol he was obtaining a substance similar to sour milk. Pasteur analyzed the chemical contents of the sour substance and found that it contained a substantial amount of lactic acid instead of alcohol. When he compared the sediments from different containers under the microscope, he noticed that large amounts of yeast were visible in samples from the containers in which alcoholic fermentation had occurred. In contrast, in the polluted containers, the ones containing lactic acid, he observed "much smaller cells than the yeast." Pasteur's finding showed that there are two types of fermentation: alcoholic and lactic acid. Alcoholic fermentation occurs by the action of yeast; lactic acid fermentation, by the action of bacteria.
Isolating the Cell's Chemical Machinery
By the end of the nineteenth century, Eduard Buchner had shown that fermentation could occur in yeast extracts free of cells, making it possible to study fermentation biochemistry in vitro. He prepared cell-free extracts by carefully grinding yeast cells with a pestle and mortar. The resulting moist mixture was put through a press to obtain a "juice" to which sugar was added. Using a microscope, Buchner confirmed that there were no living yeast cells in the extract.
Upon studying the cell-free extracts, Buchner detected zymase, the active constituent of the extracts that carries out fermentation. He realized that the chemical reactions responsible for fermentation were occurring inside the yeast. Today researchers know that zymase is a collection of enzymes (proteins that promote chemical reactions). Enzymes are part of the cellular machinery, and all of the chemical reactions that occur inside cells are catalyzed and modulated by enzymes. For his discoveries, Buchner was awarded the Nobel Prize in Chemistry in 1907 (Barnett 2000; Barnett & Lichtenthaler 2001; Encyclopaedia Britannica 2010).
Around 1929, Karl Lohmann, Yellapragada Subbarao, and Cirus Friske independently discovered an essential molecule called adenosine triphosphate (ATP) in animal tissues. ATP is a versatile molecule used by enzymes and other proteins in many cellular processes. It is required for many chemical reactions, such as sugar degradation and fermentation (Voet & Voet 2004). In 1941, Fritz Albert Lipmann proposed that ATP was the main energy transfer molecule in the cell.
Sugar Decomposition
Glycolysis — the metabolic pathway that converts glucose (a type of sugar) into pyruvate — is the first major step of fermentation or respiration in cells. It is an ancient metabolic pathway that probably developed about 3.5 billion years ago, when no oxygen was available in the environment. Glycolysis occurs not only in microorganisms, but in every living cell (Nelson & Cox 2008).
Because of its importance, glycolysis was the first metabolic pathway resolved by biochemists. The scientists studying glycolysis faced an enormous challenge as they figured out how many chemical reactions were involved, and the order in which these reactions took place. In glycolysis, a single molecule of glucose (with six carbon atoms) is transformed into two molecules of pyruvic acid (each with three carbon atoms).
In order to understand glycolysis, scientists began by analyzing and purifying the labile component of cell-free extracts, which Buchner called zymase. They also detected a low-molecular-weight, heat-stable molecule, later called cozymase. Using chemical analyses, they learned that zymase is a complex of several enzymes; and cozymase is a mixture of ATP, ADP (adenosine diphosphate, a hydrolyzed form of ATP), metals, and coenzymes (substances that combine with proteins to make them functional), such as NAD+ (nicotinamide adenine dinucleotide). Both components were required for fermentation to occur.
The complete glycolytic pathway, which involves a sequence of ten chemical reactions, was elucidated around 1940. In glycolysis, two molecules of ATP are produced for each broken molecule of glucose. During glycolysis, two reduction-oxidation (redox) reactions occur. In a redox reaction, one molecule is oxidized by losing electrons, while the other molecule is reduced by gaining those electrons. A molecule called NADH acts as the electron carrier in glycolysis, and this molecule must be reconstituted to ensure continuity of the glycolysis pathway.
The Chemical Process of Fermentation
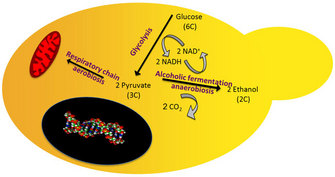
As mentioned above, glucose is converted into pyruvic acid during glycolysis. When oxygen is available, pyruvic acid enters a series of chemical reactions (known as the tricarboxylic acid cycle) and proceeds to the respiratory chain. As a result of respiration, cells produce 36–38 molecules of ATP for each molecule of glucose oxidized.
In the absence of oxygen (anoxygenic conditions), pyruvic acid can follow two different routes, depending on the type of cell. It can be converted into ethanol (alcohol) and carbon dioxide through the alcoholic fermentation pathway, or it can be converted into lactate through the lactic acid fermentation pathway (Figure 3).
Since Pasteur's work, several types of microorganisms (including yeast and some bacteria) have been used to break down pyruvic acid to produce ethanol in beer brewing and wine making. The other by-product of fermentation, carbon dioxide, is used in bread making and the production of carbonated beverages. Other living organisms (such as humans) metabolize pyruvic acid into lactate because they lack the enzymes needed for alcohol production, and in mammals lactate is recycled into glucose by the liver (Voet & Voet 2004).
Selecting Yeast in Beer Brewing and Wine Making
Humankind has benefited from fermentation products, but from the yeast's point of view, alcohol and carbon dioxide are just waste products. As yeast continues to grow and metabolize sugar, the accumulation of alcohol becomes toxic and eventually kills the cells (Gray 1941). Most yeast strains can tolerate an alcohol concentration of 10–15% before being killed. This is why the percentage of alcohol in wines and beers is typically in this concentration range. However, like humans, different strains of yeast can tolerate different amounts of alcohol. Therefore, brewers and wine makers can select different strains of yeast to produce different alcohol contents in their fermented beverages, which range from 5 percent to 21 percent of alcohol by volume. For beverages with higher concentrations of alcohol (like liquors), the fermented products must be distilled.
Summary
Today, beer brewing and wine making are huge, enormously profitable agricultural industries. These industries developed from ancient and empirical knowledge from many different cultures around the world. Today this ancient knowledge has been combined with basic scientific knowledge and applied toward modern production processes. These industries are the result of the laborious work of hundreds of scientists who were curious about how things work.
References and Recommended Reading
Barnett, J. A. A history of research on yeast 1: Work by chemists and biologists, 1789–1850. Yeast 14, 1439–1451 (1998)
Barnett, J. A. A history of research on yeast 2: Louis Pasteur and his contemporaries, 1850–1880. Yeast 16, 755–771 (2000)
Barnett, J. A. & Lichtenthaler, F. W. A history of research on yeast 3: Emil Fischer, Eduard Buchner and their contemporaries, 1880–1900. Yeast 18, 363–388 (2001)
Encyclopaedia Britannica's Guide to the Nobel Prizes (2010)
Godoy, A., Herrera, T. & Ulloa, M. Más allá del pulque y el tepache: Las bebidas alcohólicas no destiladas indígenas de México. Mexico: UNAM, Instituto de Investigaciones Antropológicas, 2003
Gray, W. D. Studies on the alcohol tolerance of yeasts. Journal of Bacteriology 42, 561–574 (1941)
Huxley, T. H. Popular Lectures and Addresses II. Chapter IV, Yeast (1871). Macmillan, 1894
Jacobs, J. Ethanol from sugar: What are the prospects for US sugar crops? Rural Cooperatives 73(5) (2006)
McGovern, P. E. Uncorking the Past: The Quest for Wine, Beer, and Other Alcoholic Beverages. Berkeley: University of California Press, 2009
Nelson, D. L. & Cox, M. M. Lehninger Principles of Biochemistry, 5th ed. New York: Freeman, 2008
Pasteur, L. Mémoire sur la fermentation alcoolique.Comptes Rendus Séances de l'Academie des Sciences 45, 913–916, 1032–1036 (1857)
Pasteur, L. Studies on Fermentation. London: Macmillan, 1876
Voet, D. & Voet, J. Biochemistry. Vol. 1, Biomolecules, Mechanisms of Enzyme Action, and Metabolism, 3rd ed. New York: Wiley, 2004
Classic papers:
Meyerhof, O. & Junowicz-Kocholaty, R. The equilibria of isomerase and aldolase, and the problem of the phosphorylation of glyceraldehyde phosphate. Journal of Biological Chemistry 149, 71–92 (1943)
Meyerhof, O. The origin of the reaction of harden and young in cell-free alcoholic fermentation. Journal of Biological Chemistry 157, 105–120 (1945)
Meyerhof, O. & Oesper, P. The mechanism of the oxidative reaction in fermentation. Journal of Biological Chemistry 170, 1–22 (1947)
Pasteur, L. Mèmoire sur la fermentation appeleé lactique. Annales de Chimie et de Physique 3e. sér. 52, 404–418 (1858)
Source: https://www.nature.com/scitable/topicpage/yeast-fermentation-and-the-making-of-beer-14372813/
0 Response to "Nad is Recycled Back to Nadh to Ensure Glycolytic Continuity"
Post a Comment